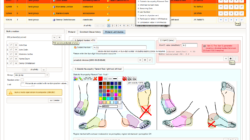
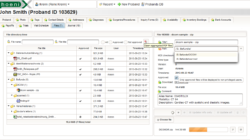
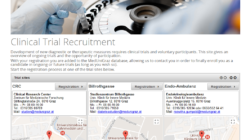
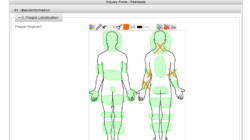
Phoenix CTMS is a modern web application combining capabilities of database software used in clinical research in one modular system.
- PRS (Patient Recruitment System)
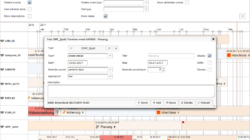
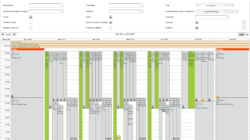
- CTMS (Clinical Trial Management System)
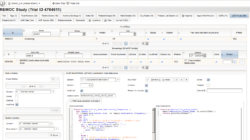
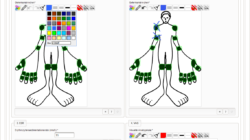
- CDMS (Clinical Data Management System) – EDC (Electronic Data Capture) Package
This unmatched feature set is geared to support all operational and regulatory requirements of the clinical front end in academic research, at CROs (Contract Research Organisations) and hospitals conducting clinical studies of any phase.
After years of collaborative development with trial sites at the Medical University of Graz, the Phoenix CTMS now becomes publicly available (LGPL 2.1). It could be the perfect choice if you e.g.
- need a private, encrypted subject registry for PII (personally identifyable information), to complement your existing CDMS
- need a solution to comply with upcoming EU-GDPR (General Data Protection Regulation)
- want to operate a secure online signup portal for subject candidates
- must not store the sensitive data in the cloud
- are not satisfied with the edit check or form field calculation possibilities available in other EDC packages
- want a CDMS that gives you unlimited Javascript form scripting support (server- and browserside)
- need to deal with large eCRFs (electronic case report forms)
- want to try a serious open source alternative for eCRFs
- need to formulate complex ad-hoc database queries to list matching subject candidates using set operations
- conduct several trials in parallel and need to organize site staff and resources
- need a software to implement various processes for ICH GCP (good clinical practice) compliance
- want a turn-key system that’s operational out-of-the-box, instead of integrating multiple systems